
2021-08-02
Indications for Use Statement
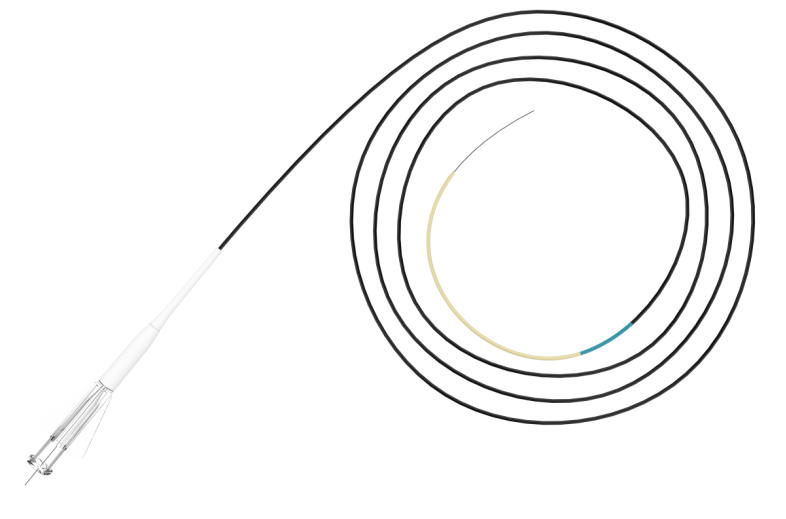
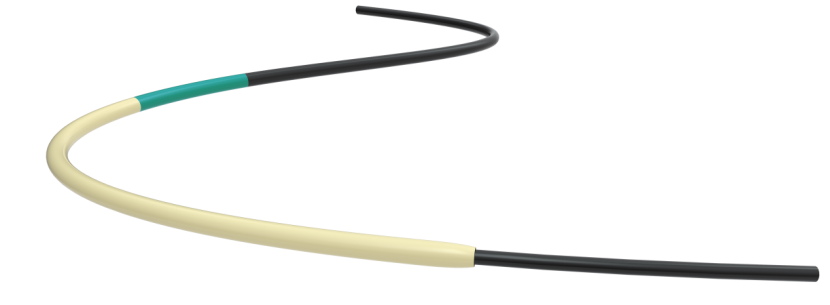
KDL Micro Catheter is used to provide support, to facilitate the placement of guidewires in the peripheral and coronary vasculature, and can be used to exchange one guidewire for another. It is also intended to assist in the delivery of diagnostics, embolic, or therapeutic materials into peripheral and coronary vessels.
About KDL Micro Catheter
510(k) Number: K201706
Regulation Number: 870.1250
Classification Product Code: DQY
Date Received: 06/22/2020
Decision Date: 07/22/2021
Decision: Substantially Equivalent (SESE)
Regulation Medical Specialty: Cardiovascular
510k Review Panel: Cardiovascular
About INT Medical
Shanghai Kindly Medical Instruments Co., Ltd. was established in 2006, a listed company on main board of Hong Kong Exchange (KDL Medical; HK1501).
We own over 1,600 employees. The headquarter is located in Jiading, Shanghai, manufacturing cardiovascular, peripheral and neuro intervention products approved by CE and FDA (510K), with a complete industrial chain of independent mold processing, product research and development, equipment development, sterilization, etc..